Organic peroxy radicals (RO2) and Criegee intermediates (CI, carbonyl oxides) are key reactive species in atmospheric chemistry, and play crucial role in the formation of secondary organic aerosol (SOA) in the atmosphere.
Recently, a research group from the Institute of Earth Environment of the Chinese Academy of Sciences (IEECAS) investigated the formation mechanisms of Criegee intermediates from the OH-initiated oxidation of ethylene (C2H4), propylene (C3H6) and 2-methylpropene (2-CH3-C3H5)) in the presence of O2 by using quantum chemical and kinetics modeling methods.
They found that the dominant pathway of the reactions HO-RO2· + ·OH is the barrierless formation of ROOOH on the singlet PES, with decomposition to RO and HO2 radicals being the lowest-energy pathway (Fig. 1). The stability of ROOOH increases with the number of methyl substituents increasing. The trioxide intermediate does not form on the triplet PES. H-abstraction from the –CHx group forming carbonyl oxides is favorable for HO-RO2 radicals.
For the reactions HO-RO2· + HO-RO·, the dominant pathway is the barrierless formation of ROOOR on the singlet PES, with dissociation back to the separate reactants being the lowest-energy pathway. The impact of the number and position of methyl substituents on the stability of ROOOR is very minor.
The barrier and endothermicity for the formation of carbonyl oxides from the self-reaction of HO-RO2 radicals significantly decrease with increasing the number of methyl substituents. The structures of HO-RO2 radicals have a strong dependence for the barrier of carbonyl oxides formation.
This study enhances the understanding of the traditional pathways involved in the transformation mechanisms of peroxy radicals and Criegee intermediates. This has significant implications for enhancing the accuracy of numerical models and for accurately assessing the impact of anthropogenic emissions on SOA formation.
This work, published in Atmospheric Environment, was supported by the National Natural Science Foundation of China (42175134 and 41805107).
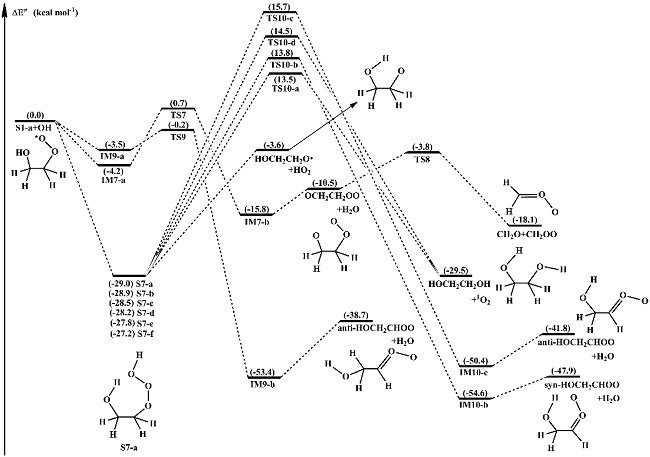
Fig. 1 Singlet PES for the reaction S1-a + •OH at the CCSD(T)//M06-2X/6-311+G(2df,2p) level (Image by CHEN, et al)
 © 2015 Institute of Earth Environment,CAS
© 2015 Institute of Earth Environment,CAS Address:No. 97 Yanxiang Road, Xi'an 710061, Shaanxi, China

 Location :
Location :