Progress in Cobalt Nanoparticles Encapsulated in N-doped Carbon for Room-Temperature Formaldehyde Removal
Formaldehyde (HCHO), a typical indoor air pollutant, has posed severely teratogenic and carcinogenic effects on human health. It is of great significance to remove HCHO and thus to improve indoor air quality.
The room-temperature catalytic oxidation technique has been recognized as an efficient approach to converting HCHO into harmless CO2 by using reactive oxygen species (ROS) on the surface of the catalysts, without any additional thermal and photon sources utilized. Abundant ROS can be generated over noble metal nanoparticles such as metallic Pt and Ir, owing to their strong ability to activate O2.
However, the high cost of precious metals impedes the wide-scale application for indoor HCHO purification. In addition, the room-temperature catalytic conversion of HCHO over conventional transition-metal oxides is restricted by their limited ability to generate ROS.
Focusing on these issues, a research group led by Prof. HUANG Yu from Institute of Earth Environment of the Chinese Academy of Sciences have previously reported the Co nanoparticles encapsulated in nitrogen-doped carbon with 85% of HCHO removal efficiency at room temperature. However, the size effect of Co particle on HCHO oxidation, and the interaction between Co core and the carbon layer, and its promotion to O2 adsorption and activation remained unclear.
Very recently, Prof. HUANG Yu’s research group prepared a series of N-doped carbon encased metallic Co (Co@NC-x) nanocatalysts to explore the effect of the Co particle size on HCHO oxidation.
The small-sized and highly dispersed Co nanoparticles were formed in Co@NC-0.25 (Fig. 1), which exhibited the HCHO removal higher than 90% and possessed the highest specific catalytic activity (Fig. 2), implying the vital importance of the size of Co particle confined in carbon for its reactivity.
The optimal Co particle size imparted the effective transfer of electrons from the metal core to the outer carbon surface, and thus leading to the enhanced oxygen activation. Density functional theory calculations demonstrated an evident charge transfer occurring from the metallic Co core to the carbon layer.
Moreover, the length of the O–O bond was elongated. These results revealed that the special Co@NC structure could supply an electron-rich carbon surface, facilitating O2 activation and HCHO conversion (Fig. 2).
This study provided a new insight into indoor HCHO purification using transition-metal nanocatalysts with a similar efficiency as precious metals. The optimized size of Co nanoparticles and the special metal@NC structure endowed the optimal utilization of active species and effective oxygen activation.
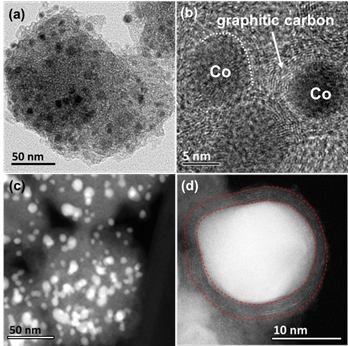
Fig. 1 The microstructures of Co@NC-0.25.(Image by ZHU, et al)
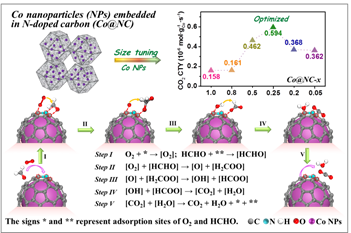
Fig. 2 The size effect of Co particle on HCHO oxidation (up) and the the conversion mechanism of HCHO over the as-prepared Co@NC-x (bottom). (Image by ZHU, et al)
This work, published in the ACS ES&T Engineering , was financially supported by the National Key Research and Development Program of China, the Strategic Priority Research Program of the Chinese Academy of Sciences, and National Science Foundation of China.
Contact: Bai Jie, Institute of Earth Environment, Chinese Academy of Sciences, Xi'an, China. Email: baijie@ieecas.cn
 © 2015 Institute of Earth Environment,CAS
© 2015 Institute of Earth Environment,CAS Address:No. 97 Yanxiang Road, Xi'an 710061, Shaanxi, China

 Location :
Location :