Nitrogen oxides (NOx, including NO and NO2) in the atmosphere are one of the important precursors of secondary aerosol formation.
The latest research has confirmed that the atmospheric chemical reaction of heterogeneous sulfur dioxide oxidation with nitrogen dioxide is an important way of sulfate formation during the current haze in China. Therefore, the development of NOx environmental control technology is of great significance to improve air quality in China.
Photocatalytic technology reduces the concentration of NOx with the help of highly oxidizing species formed by light energy excitation, and blocks the atmospheric chemical reaction way of condensing to form secondary aerosols, so as to achieve the purpose of green and efficient haze reduction, which has a broad application prospect.
The environmental pollution control group led by Prof. HUANG Yu from the institute of earth environment, Chinese Academy of Sciences, has been working on the application of nano-photocatalytic air pollution control.
Recently, the research team has focused on the adsorption-desorption behavior of thermodynamics, light absorption behavior, and photoinduced electronic-hole behavior in the process of NO photocatalysis, launched the nanomaterial surface vacancy control research, designed and developed a series of efficient nanometer photocatalytic materials.
The intrinsic anionic vacancies in semiconductors were very important for heterogeneous reactions because they could improve the light-absorbing ability while acting as specific sites for adsorption of reactant molecules and capturing photoexcited electrons. This study provided a feasible strategy for air pollution control over surface modified photocatalysts.
Herein, graphitic carbon nitride (g-C3N4) microtubes with tunable N-vacancy concentrations were controllably fabricated using an in-situ chemical method. The NO removal ratio and apparent reaction rate constant of the g-C3N4 microtubes were 1.8- and 2.6-times higher than those of pristine g-C3N4, respectively.
The results indicated that surface N-vacancies act as specific sites for the adsorption-activation of reactants and photo-induced electron capture. In-situ DRIFTS revealed that NO and O2 participate in adsorption-/photo-reactions at N-vacancies in N-deficient g-C3N4, and at terminal N-H bonds in pristine g-C3N4 (Fig. 1).
Besides, they described the synthesis of plasmonic Bi/Bi2O2-xCO3 and demonstrated the presence of surface oxygen vacancies, revealing that the maximal NOx removal efficiency of Bi/Bi2O2-xCO3 under visible light irradiation reached 50.5% and exceeded that of a commercial photocatalyst, while the production of toxic NO2 as a by-product was completely suppressed (the selectivity reached up to 98%). In-situ introduction of plasmonic Bi on the surface of Bi2O2-xCO3 promoted the generation of H2O2 by capturing electrons from the defect states of Bi2O2-xCO3 via the two-electron reduction of O2 and thus inhibited NO2 production, additionally broadening the light absorption range of the above photocatalyst (Fig. 2).
In addition, the wide optical response range and high oxidation ability are essential for photocatalytic application in air pollution control. They also proposed a one-step hydrothermal method for in-situ deposition of nitrogen-doped carbon quantum dots (NCDs) on the surface of the hollow ZnSn(OH)6 cubes. Through the regulation of composition and morphology, the ternary Z-scheme catalysts showed significantly enhanced visible and near-infrared light-driven (VIS-NIR) photocatalytic activities for nitric oxide (NO) removal. This work proposed two different photo-induced electron activation pathways. The constructed ternary SnO2/NCDs/ZHS Z-scheme nanohybrid not only facilitated the redox interaction to accelerate oxygen activation and maximized the redox capacity for nanohybrids, but also remarkable enhanced the NO removal efficiency and high nitrogen dioxide (NO2) selectivity under VIS-NIR irradiation (Fig.3).
More generally, these works indicated an effective strategy to design photocatalytic nanomaterials with strong redox capacity, high selectivity and wide-range absorption for environmental remediation.
This research was financially supported by the National Key Research and Development Program of China (2016YFA0203000) and the National Science Foundation of China (Nos. 41573138 and 51878644).
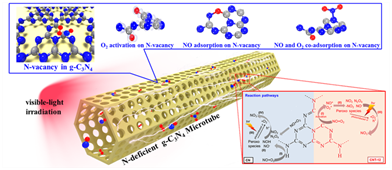
Fig. 1 Photocatalytic mechanism for NOx removal over N-deficient g-C3N4 microtube (Image by HUANGY et al).
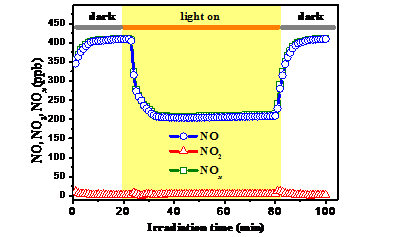
Fig. 2 The relative change NO, NO2 and NOx concentration as a function of irradiation time in the presence of B/BOC-2 under visible light irradiation. (Image by HUANGY et al)
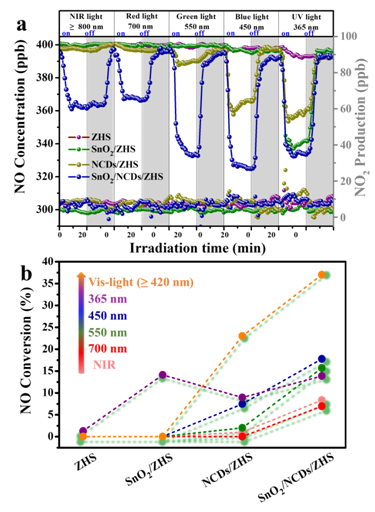
Fig. 3 Time dependence of NO removal activity (d) and the corresponding NO conversion ability (e) of ZHS, SnO2/ZHS, NCDs/ZHS and SnO2/NCDs/ZHS samples irradiated at different wavelengths. (Image by HUANGY et al)
Contact: Bai Jie, Institute of Earth Environment, Chinese Academy of Sciences, Xi'an, China. Email: baijie@ieecas.cn
 © 2015 Institute of Earth Environment,CAS
© 2015 Institute of Earth Environment,CAS Address:No. 97 Yanxiang Road, Xi'an 710061, Shaanxi, China

 Location :
Location :