Formaldehyde is a typical indoor air pollutant with teratogenic and carcinogenic effects. Conventional adsorption/absorption technique for HCHO removal is limited by secondary pollution, and it is difficult to meet the national indoor air quality standards in practical application.
Comparably, room-temperature catalytic oxidation spurns the above drawbacks and could selectively decompose HCHO into harmless CO2 without generation of harmful by-products even at ambient temperature. It has been proven to be a promising method for indoor HCHO removal.
Even though the traditional transition-metal oxides catalysts can completely decompose HCHO at relatively low temperature(60~80 ℃), the HCHO removal efficiency would be largely restricted with the treatment temperature decreased to room temperature.
For the temperature limitations of formaldehyde fully catalytic oxidation, Prof. HUANG Yu and his research team from Institute of Earth Environment of the Chinese Academy of Sciences, developed a series of room-temperature catalytic materials recently via constructing phase interface and engineering specific facets, and revealed the influence on intermediates production and reaction mechanism.
The oxidation of formaldehyde into formic acid is one of the rate-determining steps during formaldehyde oxidation reaction. Given the important role of molecular O2 activation on the oxidation of formaldehyde, Prof. HUANG and his research team developed Co nanoparticles encapsulated in nitrogen-doped carbon (Co@NC). The material showed high and stable efficiency on formaldehyde removal at room temperature, and its catalytic performance is less affected by humidity (Applied Catalysis B: Environmental 2019, 258,117981).
In addition, they successfully synthesized nano-zirconia tetragonal-monoclinic phase junction(TMZ), which effectively promoted the activation of interfacial molecular O2. A new mechanism of D-H and Mvk pathways of room-temperature catalyzing HCHO oxidation at the interface of TMZ phase junction was proposed based on the DFT calculations and in-situ DRIFTS measurements. Its rate-determining step was the transfer of H atom in HCHO to the lattice O atom in O-Zr bond of TMZ. This work enriched the study on reaction path of formaldehyde oxidationat room temperature and provided theoretical support for the design of catalytic materials for room-temperature removal of HCHO (Chemical Engineering Journal 2020, 380, 122498).
Furthermore, the team in collaboration with Prof. LEE Shun-cheng from the Hong Kong Polytechnic University, tailored the exposure of three major facets of MnOx-CeO2 (MCO) and systematically unraveled their effects on the formation and quantitative effect of oxygen vacancy, catalytically active zones, and active-site behaviors (Environmental Science & Technology, 2019, 53, 18, 10906-10916).
At present, those series of highly efficient formaldehyde purification materials have achieved large-scale production, making it possible to realize fully catalytic oxidation of indoor formaldehyde at room temperature under the condition of purifier.
Relevant research, published in Applied Catalysis B: Environmental, Chemical Engineering Journal, and Environmental Science & Technology, was jointly supported by the National Key Research and Development Program of China (2016YFA0203000) and the National Science Foundation of China.
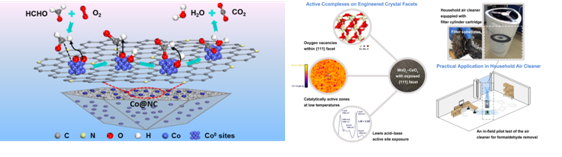
Fig.1 Schematic illustration and reaction pathway of catalytic-oxidation of HCHO on the Co@NC catalyst at room temperature (left); Active complexes on engineered crystal facets of MnOx–CeO2 and scale-up demonstration on an air cleaner for indoor formaldehyde removal(right). (Image by HUANG Yu et al.).
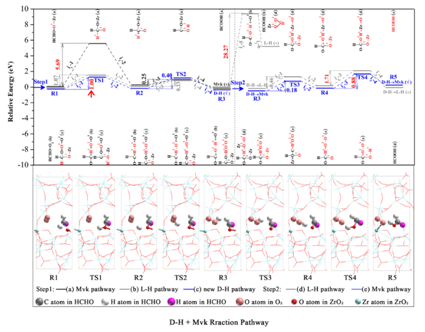
Fig.2 D?H and Mvk reaction pathway of HCHO transformed into HCOOH at the ZrO2 tetragonal-monoclinic phase junction interface (Image by HUANG Yu et al.).
Contact: Bai Jie, Institute of Earth Environment, Chinese Academy of Sciences, Xi'an, China. Email: baijie@ieecas.cn
 © 2015 Institute of Earth Environment,CAS
© 2015 Institute of Earth Environment,CAS Address:No. 97 Yanxiang Road, Xi'an 710061, Shaanxi, China

 Location :
Location :