Non-traditional stable isotope geochemistry is an emerging direction in the field of geosciences, which has developed rapidly in recent years. Boron (B) isotopes are known for theirs “δ11B-pH proxy” technology. It was used the B isotope composition of marine bio-carbonate (coral, foraminifera, etc.) to reconstruct seawater pH and atmospheric CO2 at that time.
Thermal ionization mass spectrometry (TIMS) and multi-collector inductively coupled plasma mass spectrometry (MC-ICP-MS) are the two main types of instruments for high-accuracy B isotopic analysis. MC-ICP-MS is more time efficient with no isobaric interference than TIMS. Therefore, MC-ICP-MS is becoming the preferred method for precise boron isotopic analysis.
The major analytical challenges of boron isotope measurements by MC-ICP-MS is a strong B memory effect requiring long washout time. Over the past decades, several techniques have been applied to suppress the B memory effect, including adding ammonia gas to the spray chamber, the use of direct injection into the plasma to bypass nebulization in a spray chamber, and the use of different diluents/rinse solutions (water, nitric acid, hydrochloric acid, ammonia, HF). The rinse solution is the easiest method, which is easier to operate than the other two methods. However, until now, no suitable rinse solution has been found which can eliminate the memory effect of B-isotope by MC-ICP-MS.
HE Mao-Yong, associate researcher of the Institute of Earth Environment, Chinese Academy of Sciences, and engineer DENG Li, found an appropriate rinse solution to eliminate the B memory effect from nearly 20 kinds of rinse solutions during past three years.
The results demonstrated that F-based rinse solutions (HF and NaF) were the most effective. However, using HF as a rinse solution would require high content HF (more than 0.3 M) or low content HF with the samples also in an HF medium, which required special care because HF was highly toxic. By contrast, NaF (0.6 mg·g-1 NaF in 1% HNO3 solution) was equally effective regardless of the sample acid matrix. In addition, high concentration Na solution did not affect the stability of plasma, or resulted in unexpected mass bias during the analytical courses. It only took less than 4 min to reduce B signal to blank levels using NaF rinse solution.
This protocol was applicable to a wide field of B isotopic research that required high precision and accuracy (Fig. 1).
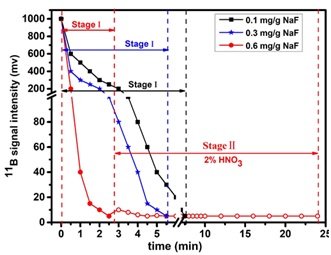
Fig.1. The 11B signal intensity during time using NaF rinse solution (the 11B signal were recorded using different rinse solution after injecting a 60 ng·g-1 boron solution for 2 min). (Imaged by HE Maoyong, et al.)
The findings were published in Journal of Analytical Atomic Spectrometry.
Contact: Bai Jie, Institute of Earth Environment, Chinese Academy of Sciences, Xi'an, China. Email: baijie@ieecas.cn
 © 2015 Institute of Earth Environment,CAS
© 2015 Institute of Earth Environment,CAS Address:No. 97 Yanxiang Road, Xi'an 710061, Shaanxi, China

 Location :
Location :